
Chaperones classified with different molecular weight.Ĭpn10, hsp10, co-chaperonin, early pregnancy factor, GroES For example, the function of many Hsp40 occurs via their actions on Hsp70. In the cell, there is complex interactions among several chaperone members to play distinct roles, as they exhibit little structural or sequence homology. Till now, 15 different groups of proteins are classified as molecular chaperones. (Table 1) The first molecular chaperone to be identified was a 60-kDa protein, which has been given the generic term chaperonin 60 (cpn60). The chaperones are classified according to their molecular weight - Hsp40, Hsp60, Hsp70, Hsp90, Hsp100 and the “small” Hsps, less than 35 kDa. Hence, the activities of housekeeping tasks and stress protection are based on their ability to interact, stabilize and protect bound polypeptides by preventing irregular interactions leading to denaturation and aggregation. Molecular chaperones perform some essential cellular functions, such as metabolism, growth, differentiation, cell to cell signaling and programmed cell death, through protein assembly and transport. In the past 20 years, it has been established that, within the cytoplasm of the cell, the molecular chaperones interact with other proteins to fold, refold or maintain the folding of the interacting proteins. The term molecular chaperone, coined by Laskey and colleagues in 1978, occurs in all organisms as they are crucial for the cell survival. Molecular chaperones are defined as “any protein that interacts, stabilizes, or helps a non-native protein to acquire its native conformation, but is not present in the final functional structure”. The proteins that are synthesised to overcome such environmental stresses have been variously called heat-shock proteins (Hsps), stress proteins or molecular chaperones, a family of abundant, evolutionarily conserved proteins that directly bind to protein substrates. These special challenges are faced by the cell & it’s organelles in the form of high protein load, molecular crowding, dynamic oligomerization etc. Key wordsĬhaperones, cellular, cytoprotective, heat shock proteins, immunity, oncogenesis IntroductionĬells in the human body are exposed to various physical, chemical, radiological, environmental and iatrogenic injuries, some of which can be withstand and/or some of it produces harmful effects. The authors reviewed, an overview of the role of chaperones, with respect to the lesions affecting oral and para-oral structures. They are potential mediators of oncogenesis as they are the key regulators of cellular growth and differentiation. Despite their advantageous effects, Hsps also has disadvantageous role in intensifying the inflammation. A tight regulation of molecular chaperones monitors the folding/misfolding, internal localization, and proteolytic turnover of proteins. It presents cytoprotective properties by the modulation of cytokine release and immunity and is involved in various physiological and pathological events. Hsps plays an essential role under most stress conditions at the cellular and subcellular levels.
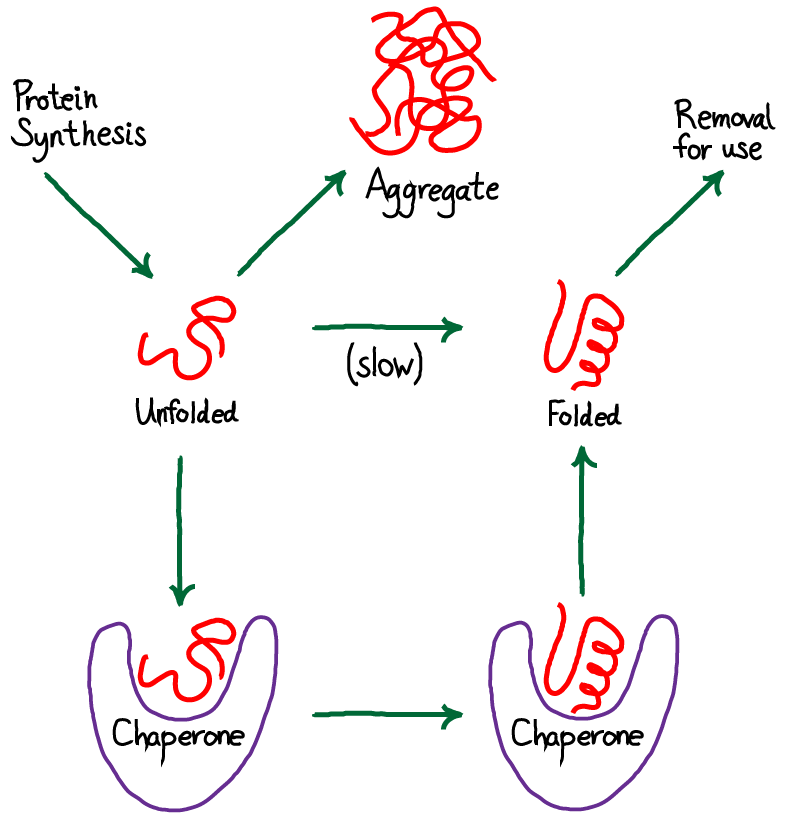
Heat shock proteins (Hsp) or the molecular chaperones are observed in most cells of humans, microbes, and in extracellular and intracellular fluids.


 0 kommentar(er)
0 kommentar(er)
